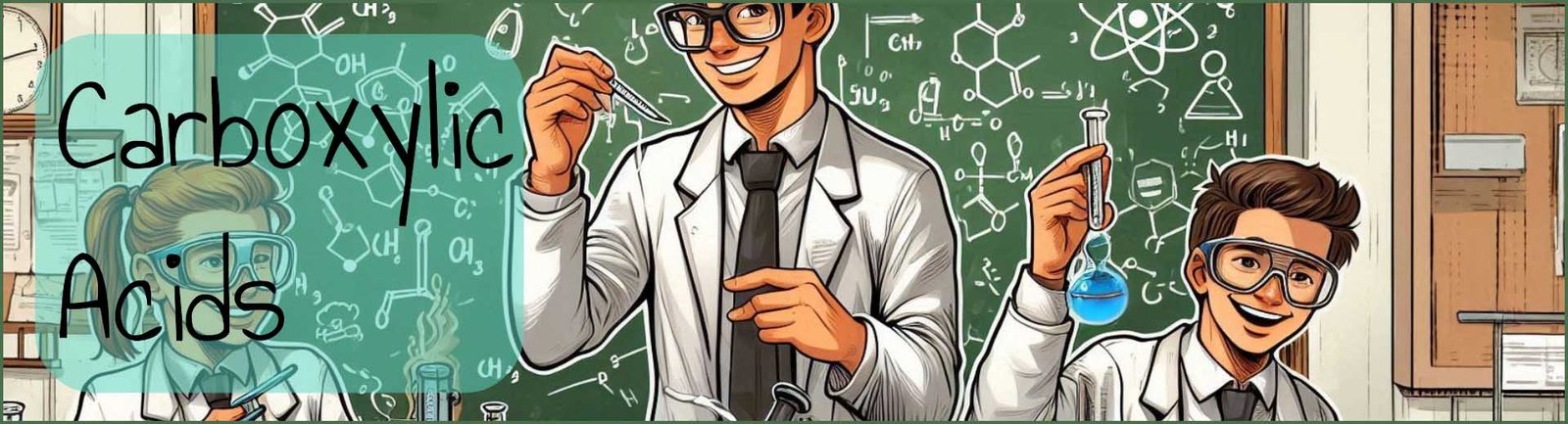

Carboxylic acids occur in a wide variety of natural compounds as shown in the image below. They can also be used to synthesise a wide variety of other biological compounds and molecules including fats and oils, proteins as well as many ester and amides. Carboxylic acids that are found naturally include methanoic or formic acid in nettle and many animal stings and bites; the smell of rancid butter is due to butanoic acid; while the smell from goats and many other animals is due to hexanoic acid. Citrus fruits, especially oranges, limes and grapefruits contain citric acid while apples contain malic acid and rhubarb leaves contain large amounts of the dicarboxylic acid ethanedioic acid.
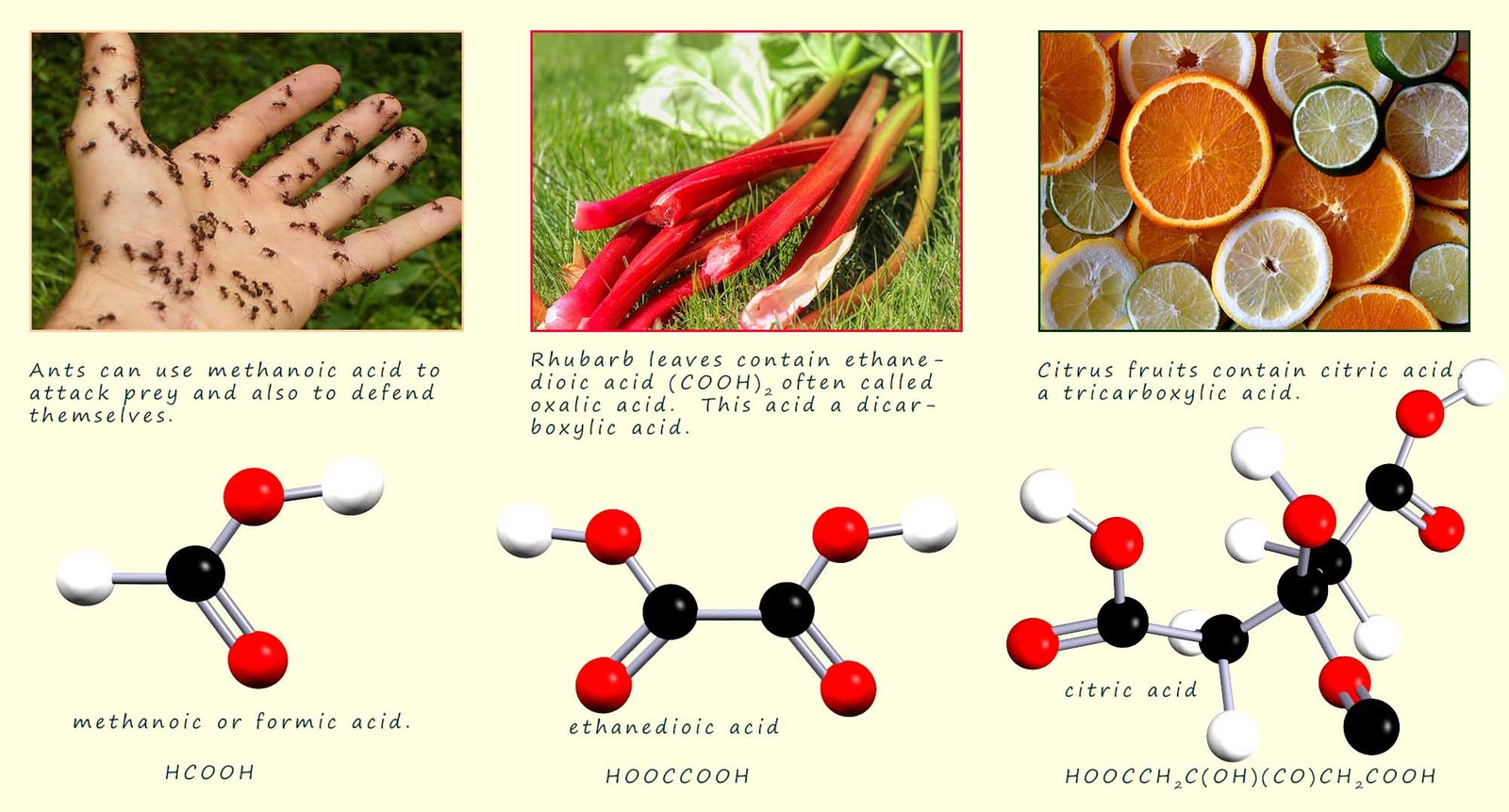
Carboxylic acids can be made by the oxidation of primary alcohols. All carboxylic acids contain the carboxyl (-COOH) functional group and are named from the corresponding alkane by simply replacing the terminal -e of the alkane by -oic. The carboxyl carbon is a high priority group and is always numbered as C1 in any carboxylic acid molecule. The general formula for a carboxylic acid is CnH2n+1COOH. The first four carboxylic acids are shown in the table below:
| alkane | carboxylic acid |
|---|---|
| methane | methanoic acid |
| ethane | ethanoic acid |
| propane | propanoic acid |
| butane | butanoic acid |
The structures of the first 4 carboxylic acids are shown below:
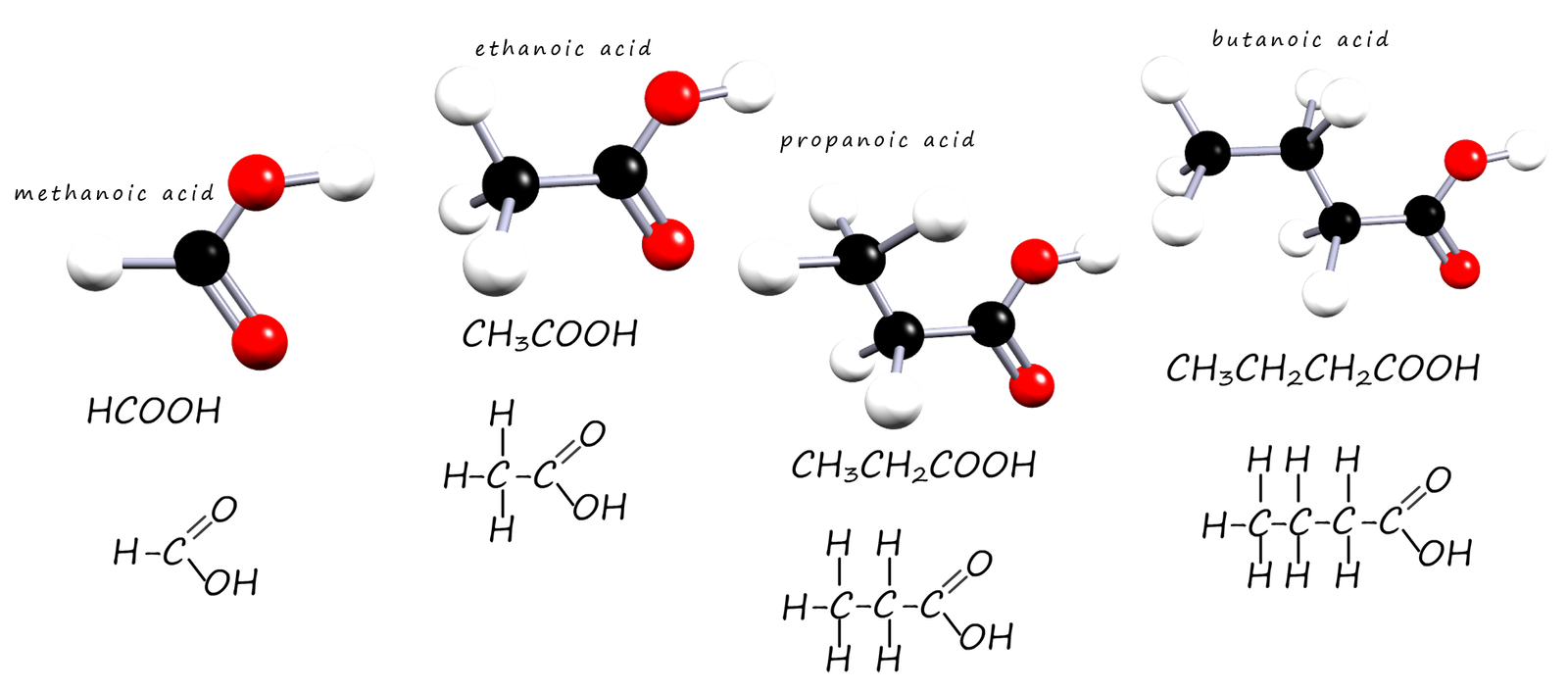
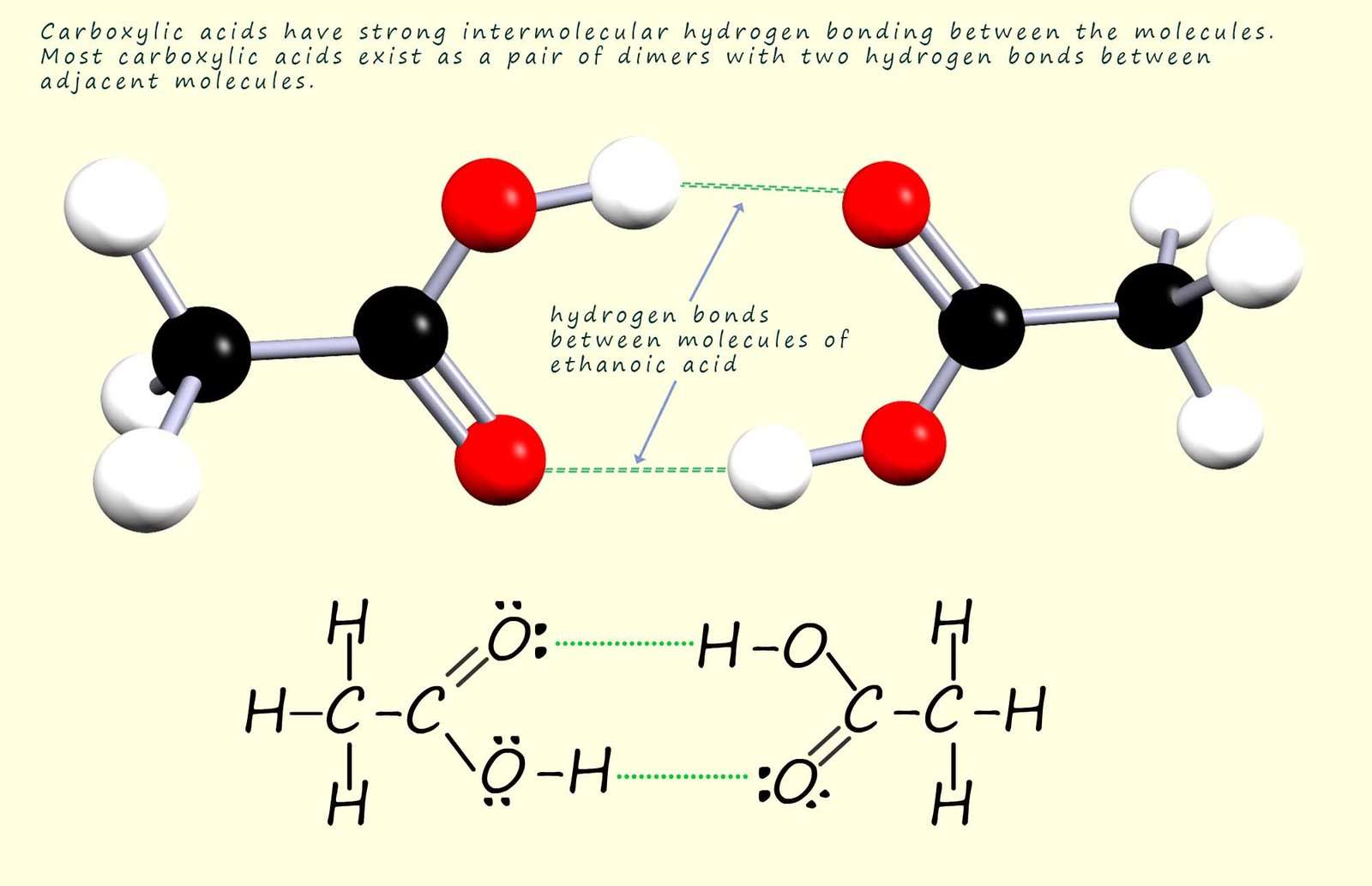
The first four carboxylic acids are very soluble in water due to the fact that the polar C=O and O-H bonds
present in the carboxyl group functional group can form hydrogen bonds with water molecules (see the diagram below). However as the chain length of
the carboxylic acid increases the solubility in water decreases, the reduction in the solubility of the carboxylic acids as the chain length increases should be expected simply
due to the presence of the larger organic non-polar portion in the molecule which will obviously not
be involved in any hydrogen bonding with water molecules.
Some of the hydrogen bonding that occurs between a carboxylic acid molecule and water molecules is shown below. Carboxylic acids with small chain lengths are water soluble due to the ability of the polar C=O and O-H bonds in the carboxyl group to form hydrogen bonds with water molecules. Hydrogen bonding will occur between the lone pairs on the carbonyl oxygen and also between the lone pairs on the oxygen atoms on the hydroxyl group and water molecules.
However the alkali metal salts of long chain carboxylic acids are soluble. This fact is often used as a method to purify long chain carboxylic acids. The long chain carboxylic acid is simply reacted with a base such as sodium or potassium hydroxide to form the soluble salt. If this salt is then acidified the carboxylic acid can then be easily extracted into an organic solvent.
It may also seem obvious that there will be strong hydrogen bonding between pairs of carboxylic acid molecules to form dimers as shown below. The presence of this hydrogen bonding between two carboxylic acid molecules results in elevated melting and boiling points compared to those you might expect from their molecular mass. For example methanoic and ethanoic acid, the two smallest carboxylic acids are liquids at room temperature and carboxylic acids with more than 8 carbon atoms in the chain are solids at room temperature.
The dissociation of a carboxylic acid in
water is shown in the image
below. Here the carboxylic acid molecule ionises to form a carboxylate anion (RCOO-) and a
hydrogen ion (H+).
It is the presence of the hydrogen ion (H+) which results in the typical reactions of
carboxylic acids with metals, metal carbonates and bases.
Carboxylic acids are weak acids; this means that very
few of the carboxylic acid molecules actually ionise, most remain intact in their undissociated molecular form.
This means that the position of equilibrium lies
very much to the left hand side; that is the reactants side and this is the reason why
carboxylic acids are weak acids with fairly high
pH values of between 3-5.

As an example consider the simplest carboxylic acid, methanoic acid (HCOOH). When methanoic acid is added to water a few of the methanoic acid molecules will ionise to form methanoate ions and hydrogen ions. Most of the methanoic acid molecules stay intact and do not dissociate or break down in water, we can show this as:
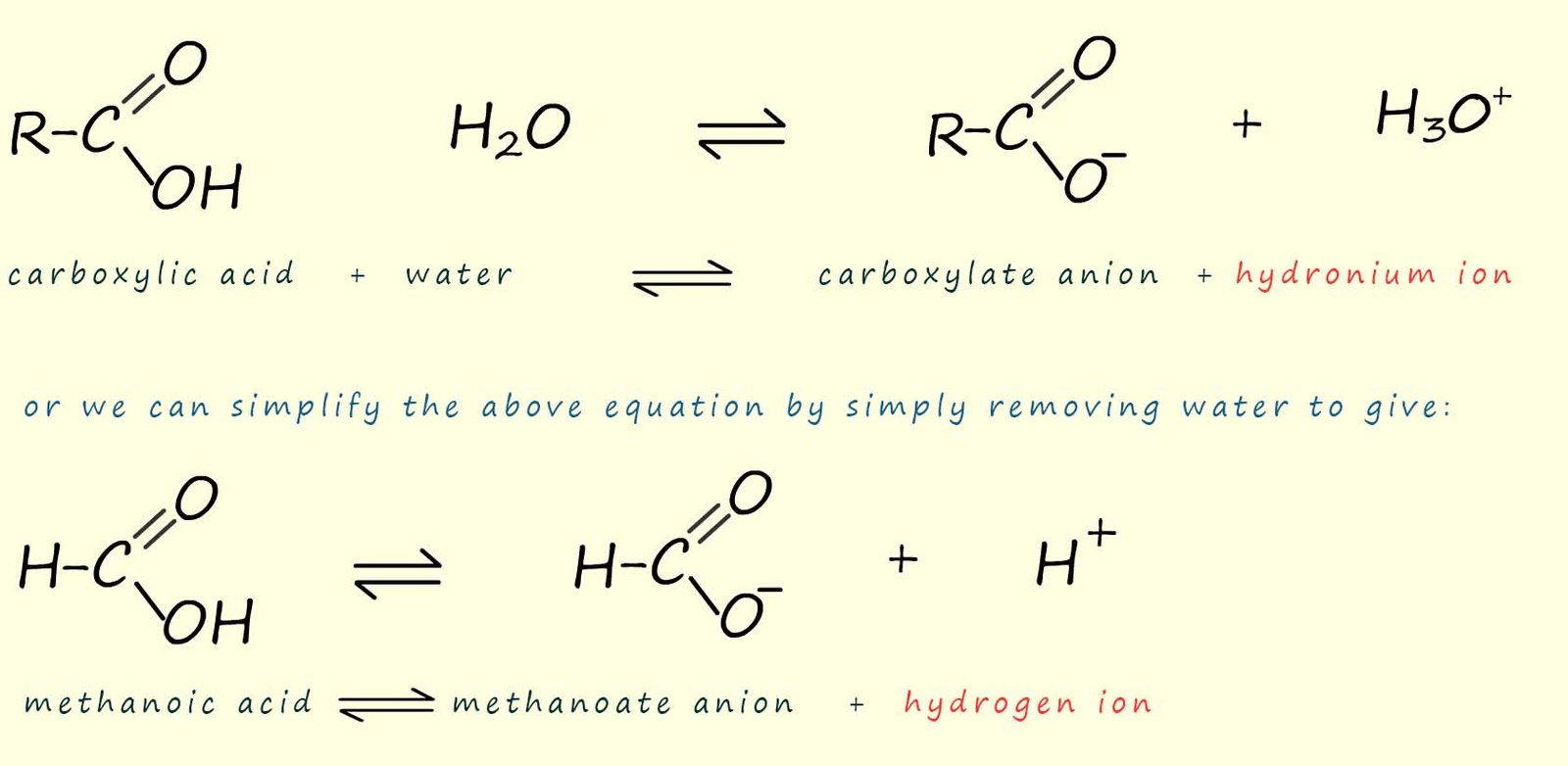
Similarly with ethanoic acid we have:
| carboxylic acid | molecular formula | ion formed | molecular formula of ion |
|---|---|---|---|
| methanoic | HCOOH | methanoate | HCOO- |
| ethanoic | CH3COOH | ethanoate | CH3COO- |
| propanoic | C2H5COOH | propanoote | C2H5COO- |
| butanoic | C3H7COOH | butanoate | C3H7COO- |
The chemistry of carboxylic acids is dominated by the carboxyl functional group. The carboxylate ion, which forms when the carboxylic acid loses a hydrogen ion (H+) is a particularly stable ion, the reason for this is simple- it is a resonance stabilised ion.
The two resonance structures for the methanoate ion are shown in the image below.
The red arrows show how the delocalised electrons move to convert one resonance form to the other.

When a carboxylic acid loses a hydrogen ion to form a carboxylate anion, this ion is much more stable than might at first be expected. This is because the carboxylate ion is a resonance stabilised ion with the negative charge spread over three atoms as shown in the image opposite. The red dotted line represents the delocalisation of an electron pair over the carbon and two oxygen atoms.
This delocalisation helps to stabilise the charge present and also helps
explain why carboxylic acids are more acidic than say alcohols or even phenols.
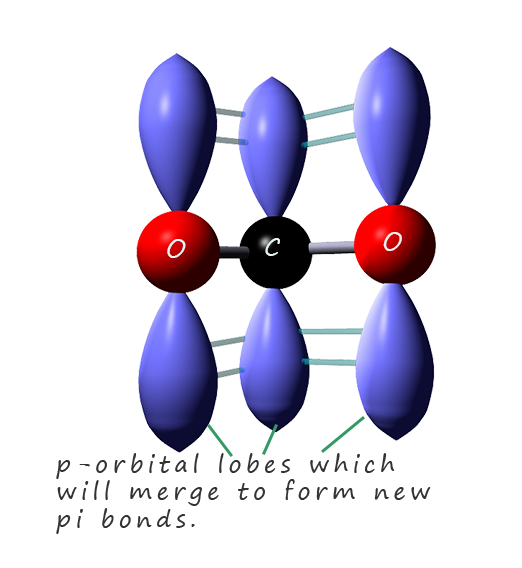
The delocalisation of the negative charge takes place across the
p-orbitals in the two oxygen atoms oxygen and the carbon atom. This is outlined in the image opposite where the p-orbital lobes are shown on each of the three atoms involved in the electron pair
delocalisation. These p-orbitals will merge to form new pi-orbitals in which the delocalised electrons will be free to move across all three atoms in the pi bond.
A reaction you have seen before is when hydrochloric acid reacts with a metal carbonate such as sodium carbonate or calcium carbonate to form a salt, water and carbon dioxide gas. Equations for these reactions are shown below:
swapping the hydrochloric acid, a strong acid for a weak carboxylic acid will produce a similar reaction but it will be much slower e.g.
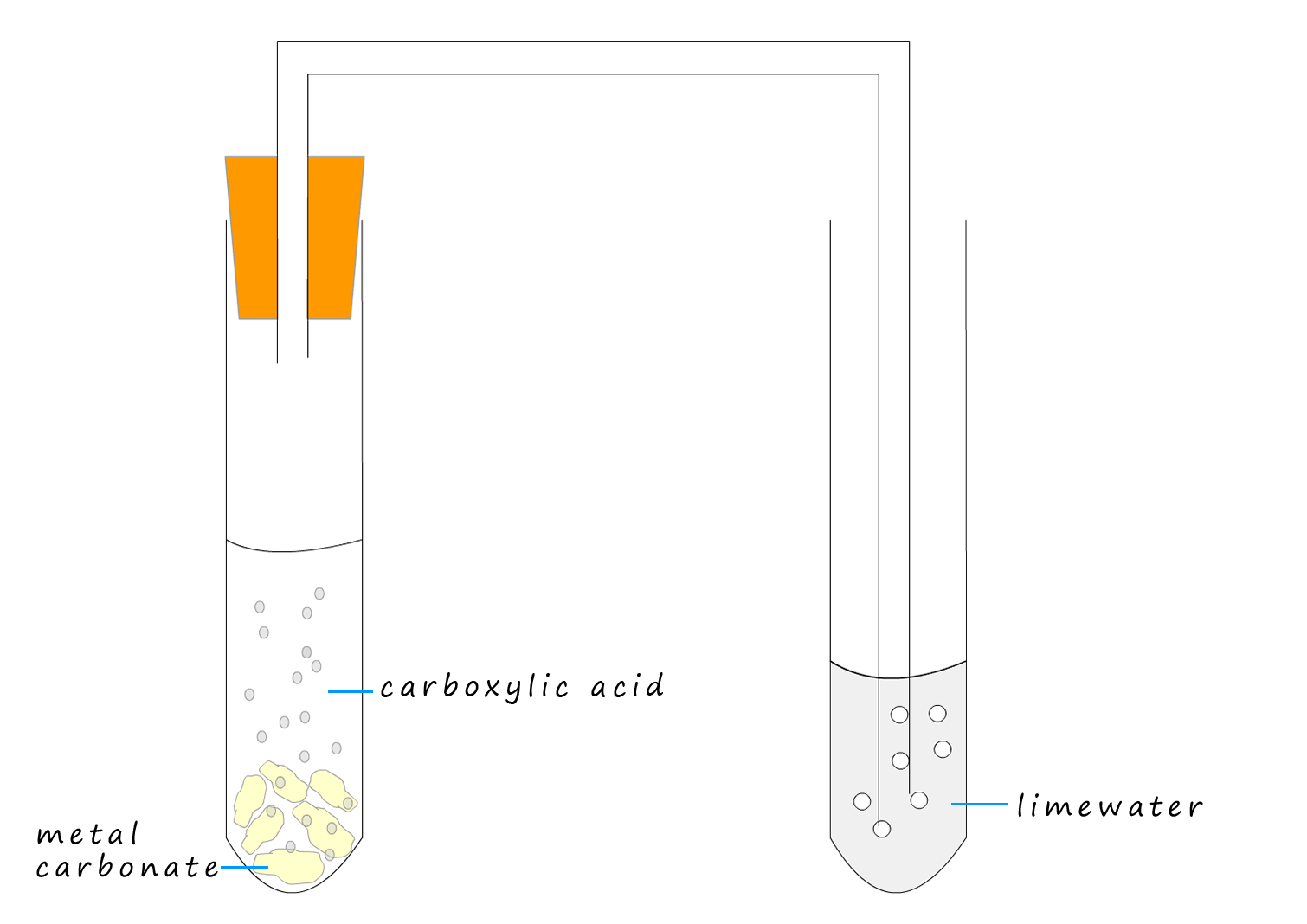
Example 2:The apparatus to compare the rate of reaction of a weak carboxylic acid and a strong acid with chalk (calcium carbonate) is shown below. Using this simple set-up we can compare the rate of reaction of a strong and a weak acid with calcium carbonate (chalk). All we have to do is count the number of bubbles or modify the apparatus to include a gas syringe and measure the volume of carbon dioxide gas released in a given time.
The reaction of carboxylic acids with metal carbonates can be use to distinguish them from much weaker acids such as phenol, which is not able to produce carbon dioxide from carbonates or hydrogencarbonate solutions.
Your chemistry teacher may demo the reactions of sodium metal with water and glacial ethanoic (acetic) acid to enable you to compare the two reactions. Glacial acetic acid is "water free" or anhydrous ethanoic acid. The name glacial comes from the fact that it forms an ice like solid just below room temperature. If two boiling tubes are set-up and a small volume of water is placed in one test tube and glacial acetic acid in the other test tube and then a pea sized piece of sodium metal is dropped into each of the two test tubes then a vigorous exothermic reaction takes place with lots of effervescence as hydrogen gas is released. In my opinion the glacial acetic acid, despite being a weak acid produces the more violent reaction simply because there is a larger concentration of hydrogen ions (H+) present in the acid than in water. An equation for this reaction with ethanoic acid is shown below:
Carboxylic acids react as you might expect with aqueous bases or alkalis to form a salt and water e.g.